Last Updated on by Mitch Rezman
A novel parrot gait called beakiation expands the locomotor range of living birds.
Researched and written by Edwin Dickinson and his team and published in January 2024, this new way of moving for parrots involves the use of their beaks while climbing on small, vertical branches under their aviary enclosures.
In an article titled, “This text will be changed accordingly”, written in the know-it-all tone of an expert who is aware of this animal.
The movie was edited in Procapture, while the two high-speed cameras also recorded side-image and backward at 125hz. They were erected to secure the data as similar as possible to the recordings taking place
Data such as contact time and stride length have been gathered throughout the lateral video footage that has been computed via ImageJ, a spatial-temporal factor.
In the background of the video existed a recognized expanse that was purposeful for synchronization purposes.
The extension between the initial touchdown and lift-off was used to assess contact time.
Stride length was speed calculated by multiplying speed by stride time.
On a known pathway, velocity was measured by following the path of the COM of each bird every sixty frames from footfall to footfall.
Beak-assisted support was carried out manually in ImageJ by reflecting one’s own proofs on the position of COM in rosy-faced lovebirds gathered through the technique of double balance-board carrying along as well.
Only trials in which there was a distinct separation of beak and hindlimb contacts were included in the single limb force analysis, where length was calculated per unit of stride time analysis.
Data were rank-transformed when needed and then examined for normality and heteroskedasticity. Each mode l included fixed effects for limb (beak vs. hindlimb) and velocity.
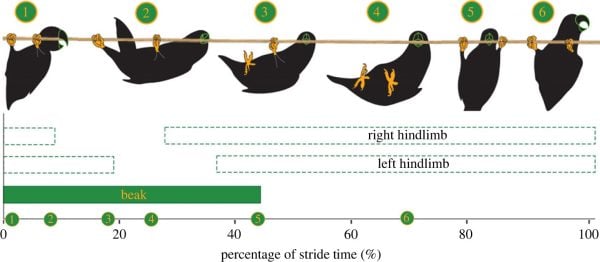
Figure 2. Representative gait diagram of beakiation within parrots, accompanied by silhouettes detailing the novel gait pattern. For display purposes, the strides begin and end with a beak touchdown. Beakiation involves an alternating gait pattern in which: (i) the beak grasps the support; (ii) the two hindlimbs release synchronously, swinging the center of mass forward while pivoting about the beak; (iii) the hindlimbs re-engage at an advanced point along the substrate; and (iv) the beak assumes a new grasping position in front of the hindlimbs.
Results were visualized and interpreted with the help of graphing software (e.g. R, GNUplot) and are shown in Table 2.
Results
The beak owned an alternating gait style of beakiation which indicated the onset of the sway cycle after the beak grasped a hold of the support; meanwhile, the hindlegs lost contact with the force substrate, and the force-generation phase occurred.
There is no overlapping support as in flying birds.
One of the main advantages of the present method is that, because the overall shape of the presence diagram is normal, so it is possible to delineate the separate regions of posterior pdfs.
The overall negative dynamics of the selected monocular force substrates E is described phenomenologically as a histogram over a forward dynamic that linear spatial statistics.
The stride length was calculated by speed*stride time and the average stride velocity was at around 0.10 m s^−1, with a stride length of 0.07±0.01 m and the average stride frequency measured 1.33±0.27 Hz, i.e. there were relationships between time on the substrate and the amount of beak did not not initate / did not produce.
The average contact time was 0.46±0.16 s (duty factor: approximately 57.70%) for the beak, is essentially higher than that of the hindleg 0.61±0.23 s (duty factor: 77.60%).
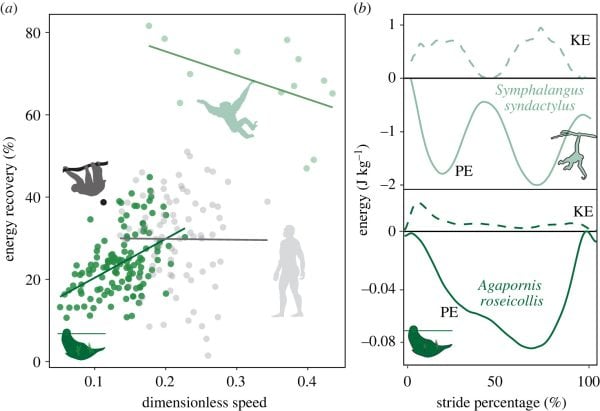
Figure 4. Bivariate plot of observed pendular period against expected pendular period in parrots (Agapornis roseicollis), brachiating non-human primates (Hylobates moloch, Ateles fusciceps, Pygathrix nemaeus) and brachiating humans (Homo sapiens). Black line indicates a perfect pendular system. All comparative data were drawn from literature values
In this study, we aim to provide the first biomechanical analysis of below-branch locomotion in parrots with a model species (Agapornis roseicollis) as well as the placement of this.
Below they delve began specialized suspensory tetrapods that may include bird swing-walk (that is a cocrcentric strategy of arboreal quadrupeds allowing the wings to be folded, potentially because of dense foliage along their pathway to cut across gaps), (i) bridging (level plane without perching) and (ii) brachiation, by human and now show an entirely original transitional gait that I refer to as beakiation. beakiation, as its name suggests, begins when an animal grabs the substrate with its beak and releases both hindlimbs simultaneously.
The bird pivots around its beak before swinging its COM forward.
The hindlegs are posterior force applied, while the beak is more acting as transferring point/ force fulcrum Figure 2.
In other words, imbicated birds’ hind legs are mounted on the force substrate at the front in the plow-down method.
Hence it is quite different from what is called beak suspension which was described in Puerto Rico as mostly been shared with beak and do not involving the rest of their body
Unlike beakiation, where both the beak and a hindlimb contribute to the movement, in beak suspension the beak is primarily doing all of the work., and yet our model species (the rose-faced could ‘olie’ without limitations his beak)
They reach an approximate average stride velocity of 0.10 m s−1 at speeds of 0.05-0.15 m s^-1; with a stride length of 0.07 ± 0.01 m; and in the test the young birds’ mean stride frequency was at the rate of 0.29 cycles / s ± 0.05 SEM (n = 12 for the following): moreover, the faster one the longer amount of body reaches more quickly on a locomotion air phase, it generated: greater than calculable awk position away from the substrate 6 Title:
This analysis was conducted with animals, not in their natural habitat. In the wild, this behavior has not ever been reported in a bird of this group. I advise not to take these results as fact.
On the other hand, we could imagine that these activities are in fact performed in their natural habitats.’ By way of examples, parrots are known to climb longitudinally from their heads to tails (Fig. 6 Avian Climbing- Concepts), to use the beak and feet to hang on and swing in branches (see Van Tets, 1985), and to rotate their body erect and hop on the ground with the beak and feet.
Pendular mechanisms during beakiation
Given the stark similarity in limb loading patterns between beakiation and brachiation, it might be assumed that parrots also adopt similar biomechanical mechanisms to gibbons and other dedicated brachiating primates.
As brachiators swing beneath branches, they effectively achieve an oscillatory motion resembling that of a pendulum. Perfect pendular systems (i.e. existing in a space devoid of air resistance or friction) are capable of transforming potential into kinetic energy so that the absolute maximum magnitudes of each energetic component are reached simultaneously (e.g. potential energy reaches its absolute minima as kinetic energy reaches its absolute maxima).
Gibbons capitalize on these mechanics, such that they achieve a near seamless exchange of potential and kinetic energy and recover nearly 80% of the initial energetic input from stride to stride.
Contrary to our predictions, however, parrots recover relatively little mechanical energy per stride, at a similar level to non-specialized brachiators like humans (approx. 25%).
There are probably a few mechanistic explanations for this discrepancy. First, beakiation occurs at significantly lower speeds than brachiation (dimensionless speeds of 0.14 versus 0.33 for beakiation and brachiation, respectively).
As such, less kinetic energy is accumulated throughout the stride. Secondly, beakiation is start-stop in nature: limiting the potential for pendular mechanical energy recovery between strides. Instead, parrots actively decelerate their COM toward the end of a stride, requiring additional muscular effort to regain their lost momentum. Though this strategy is both mechanically and metabolically expensive, it may permit more controlled and careful locomotor movements, a benefit that may be critical to successfully navigating complex and discontinuous arboreal environments [83].
Pendular period, or the time necessary to complete a full cycle (e.g. forward and back swing in a true pendulum, and touchdown to touchdown of the same point of contact in an animal) is another mechanical parameter that may be compared between locomotor modes [64,65]. Calculated from the length of the pendulum L (i.e. the distance between the pivot point and COM of object/animal, see equation above), simple gravity pendulums achieve equidistant and isochronous swings.
Parrots, like gibbons, have longer pendular periods than would be expected based on their L (i.e. distance from beak point of contact to COM), a trait probably attributable to active behavioral modulation during locomotion.
Brachiation is associated with relatively high injury rates compared with other locomotor modes, and while it remains unclear if comparable levels of injury exist for parrots during beakiation, the possible risk of injury serves as a rational explanation for why parrots and non-human primates may prioritize adaptability over efficiency when navigating challenging arboreal environments.
Thus, the deliberate, slow, and steady nature of beakiation provides parrots with more time to assess the structural integrity of a substrate and enact precise limb placement, enhancing overall stability and security while moving across slender arboreal substrates.
4.3. Broader implications
The observation of this novel behavior—which adds to a growing base of literature regarding beak-assisted locomotor strategies in birds —underscores the numerous difficulties of using morphology to predict a species’ behavioral repertoire.
While presumably facilitated by numerous adaptations (e.g. strong jaw and neck musculature, a hook-like beak), these traits are clearly not diagnostic of beakiation: many taxa show enlarged craniocervical muscles to support dietary specializations, while beak shape has been shown to covary with numerous aspects of avian ecology.
Moreover, as shown by previous anecdotal reports of beak suspension in spindalids—generalized Passeriformes that share few morphological traits with parrots—similar behaviors can be replicated via vastly different morphotypes.
Such observations emphasize the multi-dimensional nature of the avian beak as a remarkably fluid structure capable of exaptation towards numerous specialized behaviors.
Thus, only through detailed ethology —a time-consuming endeavor untranslatable to the fossil record—can biologists hope to document a more complete understanding of behavioral diversity, and from there, attempt to unravel the anatomical and physiological correlates of these activities.
Summary
Taken together, beakiation represents a novel mode of suspensory locomotion, primarily driven by the head and neck: which experience similar loading magnitudes to the forelimbs of specialized brachiating primates. Additionally, the longer-than-expected pendular periods observed during beakiation resemble patterns seen in brachiating specialists such as gibbons. However, in terms of mechanical energy recovery, parrot beakiation is characterized by lower rates of energetic recovery than seen in brachiating specialists; instead following similar trends to that observed in non-specialized brachiators like humans.
Rewritten by Mitch Rezman with permission Original Authors – Edwin Dickinson Melody W. Young and Michael C. Granatosky – Published:31 January 2024
Author Profile
Latest entries
 Feeding Exotic BirdsDecember 29, 2025How to Switch or Convert Your Bird From Seeds to Pellets: Real-Life Case Studies and Practical Guidance
Feeding Exotic BirdsDecember 29, 2025How to Switch or Convert Your Bird From Seeds to Pellets: Real-Life Case Studies and Practical Guidance Feeding Exotic BirdsDecember 16, 2025A Practical, Budget-Smart Guide to Feeding Birds Well
Feeding Exotic BirdsDecember 16, 2025A Practical, Budget-Smart Guide to Feeding Birds Well Bird EnviornmentsDecember 7, 2025Understanding Budgie Cage Bar Orientation: Myths, Realities & Practical Solutions for Vertical-Bar Bird Cages
Bird EnviornmentsDecember 7, 2025Understanding Budgie Cage Bar Orientation: Myths, Realities & Practical Solutions for Vertical-Bar Bird Cages Feeding Exotic BirdsDecember 5, 2025How Dr. T.J. Lafeber Rewrote the Future of Pet Bird Nutrition
Feeding Exotic BirdsDecember 5, 2025How Dr. T.J. Lafeber Rewrote the Future of Pet Bird Nutrition



